AS9100D
– Quality Management Systems –

Introduction to AS9100D
Maintaining superior product quality is always a constant challenge. Effective quality management requires a robust quality system. The quality system has to be designed to ensure not only good internal quality but supplier quality as well. When the supplier is in a different country or continent managing product quality can become even more challenging. To ensure quality of goods, regardless of the supplier’s home country, there are ISO standards for developing and maintaining quality systems. However, the risk of poor quality differs from industry to industry. If a part fails on a home appliance, in most cases it is likely a mere inconvenience to the customer. In contrast, if a part fails on a piece of equipment in the aviation, space or defense industry the effect could potentially cause severe injury or loss of life. The importance of quality components within these industries cannot be overstated. To ensure adequate levels of quality and customer satisfaction in the aviation, space and defense industries, the SAE AS9100 standard was developed. This standard defines the quality management system requirements to be used at all levels of the supply chain by suppliers from around the globe.
What is AS9100D
The AS9100D standard specifies the Quality Management System requirements for organizations that design, develop or manufacture aviation, space, and defense products. The AS9100 standard is an SAE document developed by the International Aerospace Quality Group (IAQG). The IAQG is comprised of representatives from Europe, the Asia Pacific region and the Americas. The standard is acknowledged worldwide. While some countries utilize various numbering practices the standard remains the same around the world. The AS9100 includes not only the ISO 9001:2015 requirements, but also identifies additional requirements specifically for the aviation, space and defense industries. Some of the changes to Revision D of the AS9100 standard include the addition of information regarding the handling of counterfeit parts, attention to the human factors of manufacturing quality, a focus on product safety, ethics training, and the process approach including SIPOC and PDCA tools, Risk Based Thinking, and monitoring supplier delivery performance. Organizations whose Quality Management System meets the requirements of the AS9100 Rev.D standard may apply for their AS9100 certificate of compliance.
To support the proper application of the AS9100 Rev. D standard, the IAQG have produced the 9100:2016 Series Clarifications publication. This document provides additional information intended to clear up any confusion or settle any disputes regarding the application of the standard. The clarifications publication includes information applicable to the 9100, 9110 and 9120 standards.
Why Implement AS9100D
Whether your organization is new to the aviation, space or defense industries or a long-time manufacturer, gaining AS9100D certification will benefit your organization. Achievement of an AS9100D certification indicates to your current and potential customers that your organization is devoted to supplying the highest level of quality and product safety. Maintaining the highest levels of quality in any industry is fundamental to the survival and growth of an organization. In order to not only achieve but also maintain consistent high quality, an organization needs a robust, well maintained quality management system QMS. Implementation of an AS9100D QMS will produce many benefits including but not limited to:
- Improvement of quality processes and meeting your customer’s needs
- Promoting decision making based upon evidence and data
- Monitoring of processes to improve efficiency, and productivity
- A Reduction in the three forms of waste and scrap costs.
- Supporting and encouraging a culture of continuous improvement
- Active risk assessment of designs and processes and effective mitigation practices
- Consistent compliance to regulatory, safety and reliability requirements.
- Enhancement of your corporate image in the global marketplace.
In addition to the many benefits to your organization, there is also the subject of corporate responsibility to consider. These industries rely heavily on their suppliers to provide material and components that not only meet their functional requirements but also safety requirements. Failure to maintain the highest levels of quality or properly identify and mitigate risk in the design of a component or assembly could have a detrimental effect on your organization or worse. Potential component or system failures could result in injuries or possible fatalities. Organizations must be diligent in identification and evaluation of risk in their designs and follow through with effective measures to reduce or eliminate that risk. Development and implementation of an AS9100D compliant QMS is an important step toward success and can have a huge effect on your bottom line.
How to Implement AS9100D
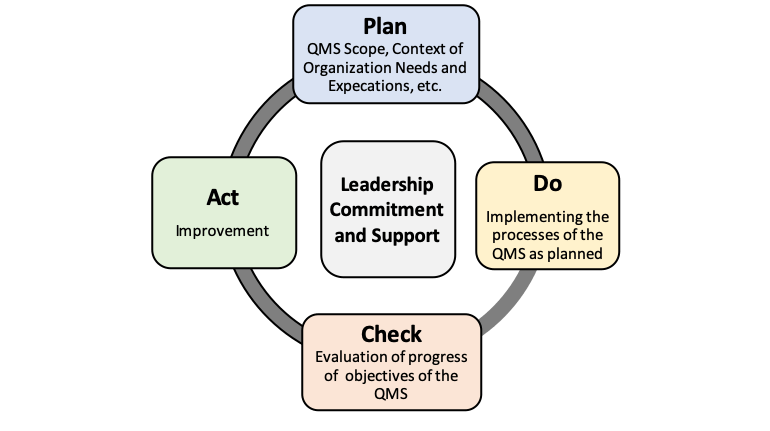
Implementation of any new management system requires dedication of management to the success of the project along with time and resources to accomplish the goal. It is the same with the successful development, implementation and maintenance of an AS9100 D quality management system. In this section, we will briefly cover the main requirements of the QMS requirements. The standard includes the requirements of ISO 9001:2015 with additional requirements unique to the aviation, space and defense industries. The standard requires the process approach based on the PDCA (Plan, Do, Check, Act) method for product and process improvements and problem resolution. In addition, the standard advocates the practice of risk based thinking. Proactive risk identification, assessment, and mitigation methodology is required. Addressing of risk is one of many items included in the previously mentioned clarification document.
The success of the QMS requires a commitment of time and resources from all levels of the organization. Top management must lead the effort towards the successful implementation and continued support of the QMS. The AS9100D standard provides a model for the establishment and implementation of an effective QMS. The following paragraphs provide brief descriptions of the main sections that constitute the body of information within the standard.
Section 4 – Context of the Organization
The AS9100D standard requires evaluation of the internal and external influences relevant to organization’s purpose or direction that could affect meeting the requirements and goals of the QMS. The organization must also:
- Understand and take into account the needs and expectations of interested parties.
- Determine and document the scope of the QMS
- Commit to the establishment, maintenance and continuous improvement of the QMS.
Section 5 – Leadership
The AS9100D standard requires that all levels of management within the organization embrace and commit to the success of the QMS. The leaders of the organization must:
- Be accountable for the success and support of the QMS
- Integrate the QMS into the organizations regular business processes
- Ensure that all product, safety, and regulatory requirements are defined and understood.
- Promote risk based thinking and use of the process approach
- Establish a quality policy and objectives aligned with corporate strategy
- Ensure that the quality policy is available for review and maintained
- Provide adequate resources for implementation and continued support of the QMS
- Encourage associates to become engaged in the QMS and contribute to its success
- Foster a culture of continual improvement within the organization.
Organizational Roles Responsibilities and Authority
Top management of the organization must assign responsibility and authority for supporting, reporting and maintaining the QMS. Furthermore, leadership of the organization is required to assign responsibility and authority for ensuring that the QMS conforms to AS9100D requirements and for regularly reporting the QMS performance results.
Section 6 – Planning
Just like any other endeavor, the development and implementation of an AS9100 compliant QMS requires proper planning. During the planning phase, top management must consider multiple factors. Management must consider the context of the organization along with any and all interested parties. In addition, management must determine the scope of the quality management system, and take proactive measures to ensure the QMS is capable of achieving its desired objectives. Plans must be developed and implemented to provide sufficient support and resources necessary to achieve continual improvement of the QMS. The AS9100 standard outlines many requirements to be met during the planning phase including but not limited to the following:
Actions to address Risks and Opportunities
The organization must develop plans for identifying, and addressing potential risks and opportunities for improvement. The plan must include methods for addressing risks and taking full advantage of opportunities for improvement. In addition, the organization must determine how to properly integrate these methods into the heart of the QMS.
Addressing Risk
There are multiple options an organization can consider for addressing risk. Many are spelled out in the standard for addressing risk. Some examples given are as follows:
- Avoiding Risk
- Taking a risk in pursuit of an opportunity
- Determining and eliminating the cause of the risk
- Taking action to change the outcome or lower the severity of the risk
- Making an educated decision to live with the risk
Addressing Opportunities
Opportunities can present themselves in a myriad of ways or forms. Taking action to seize these opportunities can have a profound impact on the organization. Stepping up when opportunity presents itself can lead to:
- Adoption of new methods and practices to improve quality
- Development and introduction of new products or product lines
- Increased market share or moving into new markets
- Utilizing new technology within the organization
Addressing risks and opportunities can improve the organizations ability to meet or exceed customer expectations of performance or quality. In addition, proactively addressing risk and seizing opportunities for improvement can also have a positive impact on your organization’s bottom line.
Establishing Quality Objectives and Plans for achieving them.
The establishment of quality objectives is vital if your QMS is going to continually improve. Quality objectives are one type of KPI (Key process indicator) that can be measured and monitored throughout the year. The quality objectives must address what needs improvement. Do your due diligence, collect and analyze data. Then let data speak for itself, not opinion or rhetoric. Quality objectives should also align with your organization’s strategic long term plans. In this section, we shall be using the terms objective and goal interchangeably. When determining the goals or objectives for the QMS, be certain that they are SMART goals.
- Specific – Define the goal as clearly as possible. Cover the why, what, when, where, and who details when defining the objective. Make sure everyone is on the same page.
- Measurable – Make certain that your goals are expressed in measurable terms. Define the measurable in the how many, how much or how often terms. Such as the goal of reducing customer shortages by 50%. A goal must be measurable because what you cannot measure you cannot improve.
- Attainable – Ensure that the goal is achievable. Make certain it is within reach and not unattainable. In addition, the goal must be accomplished by the person or persons assigned within their regular responsibilities and authority.
- Relevant – Ask yourself questions such as “Does the goal fit the organization’s needs?” “Will achievement of this goal contribute to the short- and long-term strategy of the organization?” Make sure the goal is relevant to your business.
- Time Based – Goals should have a defined timeline. For example, achieving a reduction in customer shortages by 50% within the next 12 months. This will instill a sense of urgency and enable the responsible parties to plan and manage their time accurately.
Once the goals or objectives have been determined, it is vital that they are well communicated within the organization. They are not intended to be a secret plan. In addition, the involvement of each team or individual should be clearly communicated and understood. If the goal is to reduce customer shortages by 50% then the shipping department should be involved from the start. And each associate working in the shipping department must fully understand their role in achieving the objective. Associates that are well informed and actively involved tend to take mental ownership of the process or objective. Proper communication, employee involvement and empowerment at all levels of the organization frequently results in increased employee morale and lower turnover rates.
Section 7 – Support
Successful development, implementation and continued improvement of an AS9100D compliant QMS is highly dependent upon the support received from the management team of the organization. AS9100D compliance requires top management within the organization to commit adequate resources for establishing, implementing, maintaining and driving continual improvement of the Quality Management System. The organization must consider the capabilities of and any constraints of available, qualified internal resources. In the same manner, the resources that will be acquired from external sources must be considered as well. Some examples of the resources management is required to supply are listed below. The list is not intended to be fully comprehensive but includes information relating to the major requirements needed to meet the standard.
- General Requirements – The organization must determine and meet the personnel requirements mandatory to implement and manage the QMS, and for the support of its processes. In addition, management must determine and provide the proper infrastructure, environment, monitoring and measurement resources, and measurement traceability to support a robust QMS.
- Competence – The organization must determine the level of competence required for each position supporting the QMS including the level of education, training or experience required. It is not uncommon for AS9100D compliant organizations to develop and maintain bios for each of the key personnel responsible for the QMS and its adherence to the standard.
- Awareness – Management must ensure that the persons performing work are aware of the quality policy, the objectives as mentioned in section six of the standard and roles and responsibilities to the success and continuous improvement of the Quality Management system.
- Communication – Management of the organization shall determine and establish the appropriate internal and external lines of communication required for the success of the QMS. The organization must also define the method of communication along with what will be communicated, when to communicate, whom to communicate with and how often communication will take place. Furthermore, communication should include internal and external feedback pertaining to the Quality Management System status and its objectives.
- Documentation – The organization must develop and maintain documented information required by AS9100D standard. Along with any information deemed necessary by the organization for the success of the QMS. The required documentation shall have adequate protection of the content and control revisions ensuring that any changes or updates are identified and traceable. The proper documents must be available for use where and when required. Furthermore, the document control system must provide for proper access, distribution, storage, retention and the eventual disposition of obsolete documents.
Section – 8 Operation
Top management of the organization must plan for the operation of the Quality Management System. Once the QMS is developed and implemented, the day to day operation of the QMS must be managed and monitored to ensure it is fulfilling its intended purpose and pursuing the appropriate objectives. The AS9100D standard outlines what the organization must do to ensure proper operation of the QMS. In this section, we will briefly touch on the main points contained within the standard.
- Operational Planning and Control – Plans shall be developed for the implementation and control of the processes identifying and addressing operational risk, product identity, traceability and product safety. One additional requirement included in the AS9100D standard addresses the requirements concerning the control of counterfeit parts. Proper training of personnel including verification and testing, to identify counterfeit parts, along with processes for the containment and disposition of said parts is required by the standard.
- Requirements for Products and Services – The organization is required to implement processes to ensure that all product and service requirements are met on a consistent basis. The processes for communication with customers and the criteria for acceptance of products and services are among the requirements that must be defined by the AS9100D standard.
- Design and Development of Products and Services – Compliant organizations are required to establish and support a robust design and development process to ensure product and service requirements are met. This includes but is not limited to design inputs, development and verification of the design and control of the outputs such as drawings, part lists, the material and any special product or process requirements.
- Control of Externally Supplied Processes, Products and Services – Externally sourced parts or services must be monitored and controlled. The organization should have a robust supplier quality management process with regular evaluation of supplier performance. The organization shall determine and apply the appropriate criteria for judging the quality of an external supplier’s parts or products and the supplier’s performance.
- Production and Service Provision – Systems must be in place to effectively monitor and control multiple aspects of the production processes including but not limited to:
- Equipment, Tools and Software used for automation, monitoring, measuring or controlling production processes must be validated and properly maintained.
- Validation of special processes that cannot be monitored or accurately measured
- Material or product traceability when required to assure the quality and conformity of products or services.
- Control of process changes including documentation of the review results and the associates authorizing the change in the process or processes.
- Release of Products and Services – Systems must be in place to document conformity prior to the release of a product to the customer. The organization must be able to produce evidence of compliance including the product acceptance criteria, the person authorizing the release, and their qualifications.
- Control of Non-Conforming Outputs – The organization shall develop and maintain systems and procedures for the containment and control of any non-conforming process outputs. This would include proper documentation of the non-conformity along with the segregation, disposition and prevention of use or delivery to the customer.
Section – 9 Performance
Organizations pursuing AS9100 certification must develop and maintain effective procedures for monitoring and evaluating the performance and effectiveness of their Quality Management System. If it cannot be measured it cannot be properly controlled. Therefore, the QMS must include the proper tools, associate training and sufficient resources to monitor the progress of the QMS and the achievement of planned objectives.
- Monitoring, Measurement, Analysis, and Evaluation – The organization shall determine what needs to be monitored and measured, when it shall be done, and the method of documentation.
- Internal Audit Process – Internal audits are required to be performed at regular intervals to ensure that the QMS is meeting internal, customer and any applicable regulatory requirements. Records of the audit results shall be retained as evidence of compliance and shall be available for review.
- Management Reviews – Management shall hold QMS review meetings at regular intervals. The agenda or subject matter of the review meetings are the decision of the organization. The subject matter may include but is not limited to the following:
- Reviewing the status of actions from previous reviews
- Changes to any internal or external issues relevant to the management system
- Information about the QMS performance including audit results and progress of objectives.
- Potential risks to the QMS or opportunities for improvements.
- Review of the supplier performance KPIs
Any relevant information resulting from the meetings should be shared with workers and other interested parties. The organization shall also retain documented records of the results of the management reviews.
Section 10 – Improvement
In order for an organization’s Quality Management System to be most effective, it must continually improve. Organizations must actively look for opportunities to improve the effectivity of their QMS. They should continually work toward achieving the QMS objectives while providing products and services tailored to their customer requirements.
The AS9100 standard requires that organizations have plans in place to react to non-conformities. This should include but not be limited to containment, control, Root Cause Analysis (RCA) and Corrective Action Preventative Action (CAPA). In order to prevent the problem from becoming a repeat problem, the root cause must be identified.
- A good example is a weed in your garden. The goal of your garden is to grow and supply you, the customer, with high quality fresh produce. Weeds rob the soil of essential nutrients and moisture interfering or possibly preventing the garden from achieving the planned objective. If you merely cut down or pull the weed without identifying the root, it will be back. The same can be said about quality problems. In many cases, we end up addressing the symptom and not the true root cause. If the root cause is not determined, the issues may re-occur within the same process or elsewhere in the organization. The root cause is the true underlying or fundamental cause of nonconformity.
An action plan should be developed for implementation of the appropriate corrective actions and documenting their effectiveness. The standard also requires that the organization document the incident including the corrective actions and their effectiveness. The records must be properly maintained and available for review by management or during a third party audit.
In Conclusion
As previously stated, AS9100D incorporates the PDCA cycle approach towards building, maintaining and improving your QMS. The PDCA (Plan, Do, Check, Act) cycle includes the Plan phase that covers the planning phase and beginning stages of QMS development. The Do cycle deals with QMs implementation and operation. The Check cycle is the evaluation of the QMS effectiveness. This includes the management reviews of the QMS performance. Finally the Act phase looks at ways to integrate improvements throughout the organization and ensures the PDCA cycle never ends. Thus the depiction of the PDCA cycle as a circle and not a straight line.
The initial development and implementation of an AS9100 compliant Quality Management System may take several months and a large amount of resources. One key to success is to have participation and buy-in from all levels of the organization. Quality is everyone’s responsibility. The QMS must have adequate resources and constant support from organizational leadership.
At any time during the planning, implementation or operation of the QMS questions arise regarding the AS9100 standard. Be sure to reference the 9100-2016 Series Clarification document provided by the IAQG. Referencing this document may provide the information required to clear up any confusion regarding the application of the standard. In some cases, further clarification is needed or you may require the assistance of experienced, and highly qualified subject matter experts. When additional resources or subject matter experts are needed they are close at hand. At Quality-One we can meet your needs. Therefore, if your organization is in need of additional resources or would like more information regarding AS9100D implementation, please contact one of the professionals at Quality-One.
Learn More About AS9100D
Quality-One offers Quality Management Systems Development through Consulting, Training and Project Support. Quality-One provides Knowledge, Guidance and Direction in Quality Management Systems development activities, tailored to your unique wants, needs and desires. Let us help you Discover the Value of AS9100D Consulting, AS9100D Training or AS9100D Project Support.